01 M EGTA pH 69 19 g EGTA 15 mL DI H2O pH with 1 M KOH add DI H2O to 50 mL. The solution is acidic and its pH is less than that of 01 M HCl.

Calculate The Ph Of 0 5 Of 1 0 M Nacl Solution After Electrolysis
PH pOH 14.

Ph nacl 0.1 m. NaCl Na Cl -. So the pH for NaCl is 70 that for Na2CO3 gives CO32- HOH HCO3- OH- So this reaction is producing OH- and HCO3- OH is strong base and HCO3- a weak acid so the solution will be basic which means higher pH. How to find the pOH value if the pH of a Solution is given.
The solution is basic and its pH is more than that of 01 M HCl. 3 M NaCl 03 M Na3Citrate 1753 g NaCl 882 g. La seconda parte dellesercizio richiede il calcolo del pH della soluzione.
So you would expect that the. A HCl H 3 O H 01 M. Therefore its concentration is 10-1molL pH-log10-1-1-log10log101.
HCl is a strong acid and NaOH is a strong base. A decrease or an increase in pH gives rise to the coagulation rate approaching the rate of fast coagulation according to Smoluchowski at pH 10. X 75 x 10-6 H3O pH -log H3O -log 75 x 10-6 51.
POH -log OH - -log02 07. 01M PBS pH 74 02M stock solutions of Na2HPO4 and NaH2PO4 15M 10x stock solution of NaCl Mix stock solutions. Please select more than one item to compare.
Surheten er bestemt av konsentrasjonen av hydrogenioner altså H-ioner i løsningen. Molarity of given HCl is 01 M. PH is defined as negative logarithm of H ion concentration.
Prepare a 02 M stock solution of sodium cacodylate in water 428 g100 ml. Becuse Ka is so small x will be small compared to 01 so we can delete it from the 01 - x term. Add the following amounts of 02 M HCl per 100 ml cacodylate stock solution followed by the addition of DI to a final volume of 400 ml to obtain 005 M cacodylate buffer at the desired pH Dawes 1971.
EduRev NEET Question is disucussed on EduRev Study Group by 1234 NEET Students. The lowest coagulation rate is observed at pH 49. X2 01 56 x 10-10.
81 ml Na2HPO4 dibase 19 ml NaH2PO4 monobase 20 ml 15M 10x stock NaCl 80 ml ddH2O _____ 200 ml 01 M PBS pH 74 Remember to check the pH. PH er et mål på hvor sur en væske vannløsning er. I due ioni Na e Cl - potrebbero dare idrolisi.
Ka H3O NH3 NH4 x2 01 - x 56 x 10-10. May 042021 - At 90 degree in celsius the ph of 01M nacl aqueous solution is. Type V 10 μgmL in 015 M NaCl 10 mM Tris pH 80 containing 01 sodium azide.
M n V 01 05 02 molL. -log 01 and perform basic logarithmic maths to get the pH. Azide can be added at 002 to prevent bacterial contamination.
Determiniamo la molarità della soluzione. Ii NaCl is a salt of strong acid and strong base so it is not hydrolysed and hence its pH is 7. NaCl è un elettrolita che - in acqua - è dissociato nel seguente modo.
Select up to 4 products. 3629 matches found for 01 M NACL. De ion would not hydrolyze at alland NaCl is a nutral salt The pH of the solution would be close to 7.
HCl NaCl NaCN NH 4 Cl. A NaCl. X2 56 x 10-11.
B NaOH OH - 02 M. CH3COONa NaCl D NaCl NH4Cl CH3COONa Login Remember. Effect of pH on the coagulation kinetics of microcrystalline cellulose dispersions in an aqueous 01 M NaCl solution is studied by the flow ultramicroscopy.
To Calculate the pH of 01M HCL Solution take the negative logarithm of Hydronium Ion Concentration ie. H 3 O forkortes ofte H. NaOH er sterk base slik at disse protolyserer 100.
Stabilization Buffer 10 mL 1. HEPES is a buffer that can be used to control the pH of many solutions and this particular buffer is used in our lab to make assay buffers for fluorogenic substrate assays to measure enzyme activity in the presence of various inhibitory substances. Hvis konsentrasjonen av H-ioner i en løsning er 001 mol per liter er pH i løsningen 20.
The purpose of this protocol is to prepare a 01 M HEPES stock solution at pH 74. So the pH is 7. PH HCl -log H 3 O -log 01 1.
1 Approved Answer. Food and Beverages 1 Pharmaceutical 1 Available for Sale. Sammenhengen er at tallet 001 kan skrives som potensen 102.
HCl NH 4 Cl NaCl NaCN. 01 M NaCl solution is formed bythe hydrolysis reaction of HCl and NaOH. Løsningen er sur når pH ligger mellom 1 og 7.
Strong acids react with strong bases generally give neutral salts and N aC l is one of them. POH can be simply obtained by subtracting the pH from 14 ie. The pH of 01 M aqueous solution of NaCl CH3COONa and NH4Cl will follow the order.
Since its neutral it doesnt really affect the H and OH in water and its concentration doesnt alter the pH of its solution.
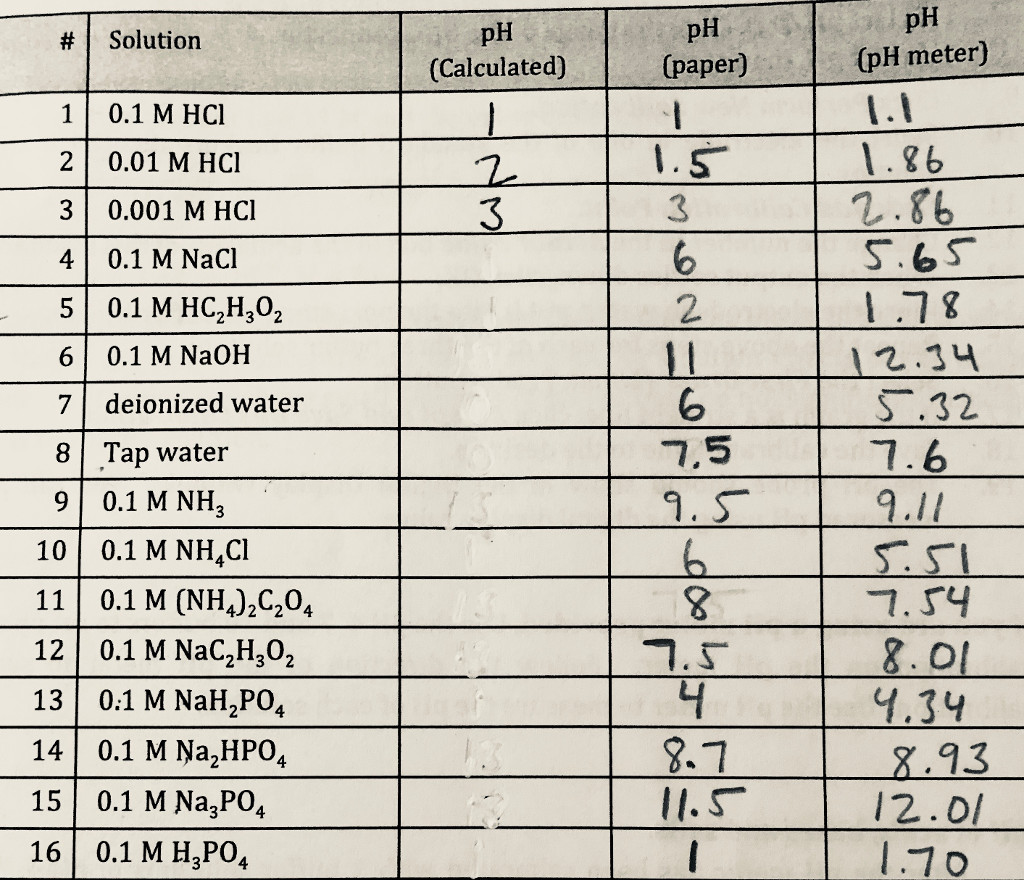
Solved How Do I Find The Ph Calculated Using What I Have Chegg Com

10 Ml Of A Solution Having Ph 4 Is Added With 990 Ml Of 0 1m Nacl Solution Ph Of Resulting Solution Brainly In

Corrosion Rate Vs Time In 1 M Nacl 0 1 M Naf Ph 8 Download Scientific Diagram

The Ph 0 1 M Solution The Following Salts Increases In The Order Youtube

A Impedance Data Recorded For Cu 37zn E In 0 1 M Nacl Ph 3 5 S In Download Scientific Diagram

Q 30 The Highest Ph Value Is Of 1 0 1 M Nacl Chemistry Chemical Kinetics 12574793 Meritnation Com

1 Acid Content Of Beverages A Titration Exercise Susb Ppt Download
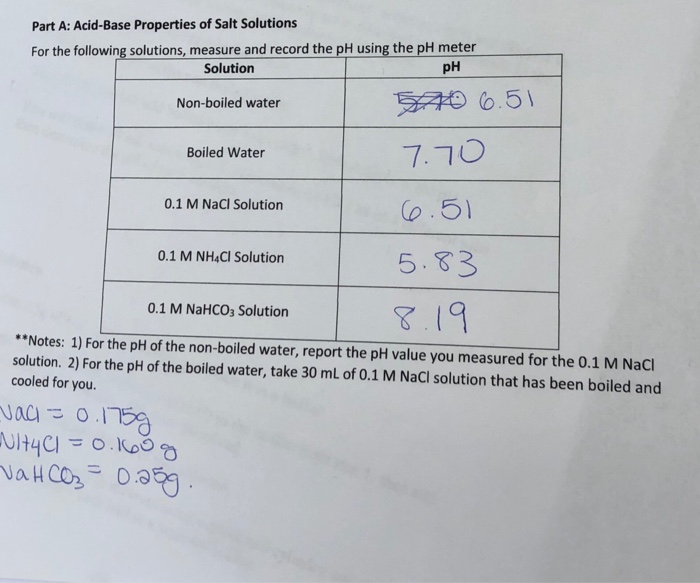
Solved Part A Acid Base Properties Of Salt Solutions For Chegg Com
Experimental Tho 2 Am Solubilities In 0 1 M Nacl At 23 C Plotted As Download Scientific Diagram
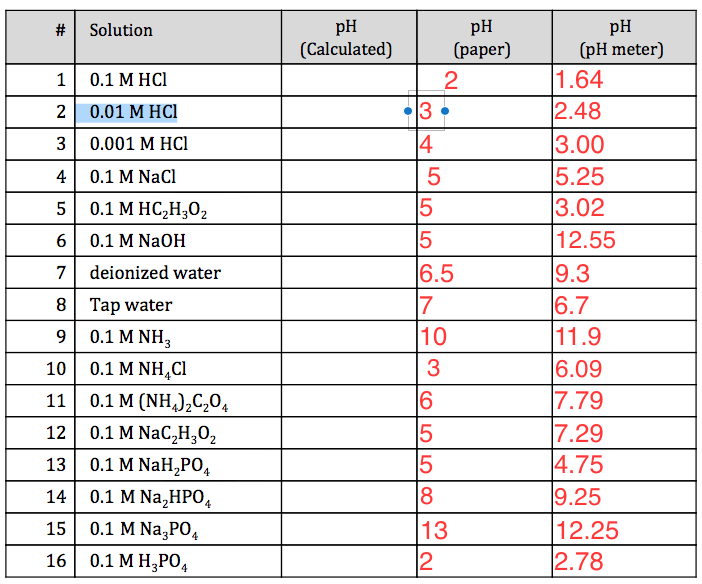
Solved Help Please 1 Explain The Reasons For The Differe Chegg Com

The Ph Of 0 1 M Solution Of The Following Salts Increases In The Order Jee 1999 A Nacl Lt Nh4cl Lt Nacn Lt Hclb Hcl Lt Nh4cl Lt Nacl Lt Nacnc Nacn Lt Nh4cl Lt
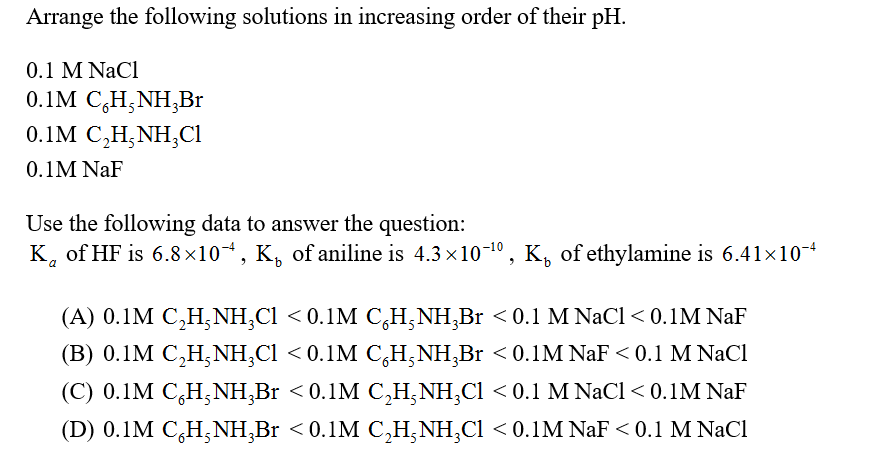
Answered 0 1 M Nacl 0 1m C H Nh Br 0 1m Bartleby

Graph Of Ph Of 0 1m Nacl Against Volume Of 0 1m Hno 3 Download Scientific Diagram

0 1 M Hcl And 0 1 M H2so4 Each Of Volume 2 Ml Are Mixed And The Volum Askiitians